What Carbon Sources Can Yeast Cells Metabolize to Make Atp From Adp Under Anaerobic Conditions?

Cellular respiration is a gear up of metabolic reactions and processes that take place in the cells of organisms to convert chemical free energy from oxygen molecules[1] or nutrients into adenosine triphosphate (ATP), and then release waste products.[2] The reactions involved in respiration are catabolic reactions, which suspension large molecules into smaller ones, releasing energy because weak high-energy bonds, in item in molecular oxygen,[3] are replaced by stronger bonds in the products. Respiration is one of the key ways a jail cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it conspicuously does not resemble 1 when it occurs in a living prison cell because of the slow, controlled release of energy from the series of reactions.
Nutrients that are commonly used past fauna and establish cells in respiration include sugar, amino acids and fatty acids, and the most mutual oxidizing amanuensis providing nigh of the chemic free energy is molecular oxygen (O2).[1] The chemical free energy stored in ATP (the bond of its third phosphate group to the rest of the molecule tin can be broken allowing more stable products to grade, thereby releasing free energy for use past the prison cell) tin then be used to drive processes requiring energy, including biosynthesis, locomotion or transport of molecules beyond jail cell membranes.
Aerobic respiration
Aerobic respiration requires oxygen (O2) in order to create ATP. Although carbohydrates, fats, and proteins are consumed equally reactants, aerobic respiration is the preferred method of pyruvate breakdown in glycolysis, and requires pyruvate to the mitochondria in order to exist fully oxidized by the citric acrid cycle. The products of this procedure are carbon dioxide and water, and the energy transferred is used to break bonds in ADP to add together a 3rd phosphate grouping to form ATP (adenosine triphosphate), by substrate-level phosphorylation, NADH and FADHtwo
| Simplified reaction: | C6H12Ohalf-dozen (due south) + half-dozen Oii (g) → 6 CO2 (g) + half-dozen H2O (50) + heat |
| ΔG = −2880 kJ per mol of CviH12O6 |
The negative ΔG indicates that the reaction can occur spontaneously.
The potential of NADH and FADH2 is converted to more ATP through an electron transport chain with oxygen and protons (hydrogen) as the "terminal electron acceptors".[one] Most of the ATP produced by aerobic cellular respiration is made by oxidative phosphorylation. The energy of O2 [1] released is used to create a chemiosmotic potential by pumping protons across a membrane. This potential is and then used to drive ATP synthase and produce ATP from ADP and a phosphate group. Biological science textbooks often state that 38 ATP molecules can be fabricated per oxidized glucose molecule during cellular respiration (two from glycolysis, 2 from the Krebs wheel, and about 34 from the electron transport system).[4] All the same, this maximum yield is never quite reached considering of losses due to leaky membranes as well equally the cost of moving pyruvate and ADP into the mitochondrial matrix, and current estimates range around 29 to xxx ATP per glucose.[iv]
Aerobic metabolism is up to fifteen times more efficient than anaerobic metabolism (which yields 2 molecules ATP per 1 molecule glucose) because the double bond in O2 is of higher energy than other double bonds or pairs of single bonds in other common molecules in the biosphere.[3] Even so, some anaerobic organisms, such as methanogens are able to go along with anaerobic respiration, yielding more ATP by using other inorganic molecules (not oxygen) of loftier energy as terminal electron acceptors in the electron transport chain. They share the initial pathway of glycolysis but aerobic metabolism continues with the Krebs wheel and oxidative phosphorylation. The post-glycolytic reactions take place in the mitochondria in eukaryotic cells, and in the cytoplasm in prokaryotic cells.
Glycolysis
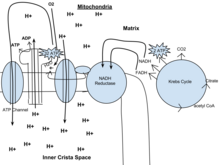
Out of the cytoplasm it goes into the Krebs wheel with the acetyl CoA. It then mixes with COii and makes two ATP, NADH, and FADH. From there the NADH and FADH go into the NADH reductase, which produces the enzyme. The NADH pulls the enzyme's electrons to transport through the electron send chain. The electron send chain pulls H+ ions through the concatenation. From the electron transport concatenation, the released hydrogen ions make ADP for an issue of 32 ATP. O2 provides most of the energy for the process and combines with protons and the electrons to brand water. Lastly, ATP leaves through the ATP channel and out of the mitochondria.
Glycolysis is a metabolic pathway that takes place in the cytosol of cells in all living organisms. Glycolysis tin can exist literally translated as "sugar splitting",[v] and occurs with or without the presence of oxygen. In aerobic conditions, the process converts ane molecule of glucose into two molecules of pyruvate (pyruvic acrid), generating energy in the course of two net molecules of ATP. Four molecules of ATP per glucose are really produced, but two are consumed as function of the preparatory phase. The initial phosphorylation of glucose is required to increase the reactivity (decrease its stability) in social club for the molecule to be cleaved into 2 pyruvate molecules by the enzyme aldolase. During the pay-off phase of glycolysis, 4 phosphate groups are transferred to ADP by substrate-level phosphorylation to make iv ATP, and ii NADH are produced when the pyruvate is oxidized. The overall reaction can be expressed this way:
- Glucose + 2 NAD+ + ii Pi + 2 ADP → ii pyruvate + 2 H+ + 2 NADH + 2 ATP + 2 H+ + 2 H2O + free energy
Starting with glucose, i ATP is used to donate a phosphate to glucose to produce glucose 6-phosphate. Glycogen can be converted into glucose half-dozen-phosphate as well with the help of glycogen phosphorylase. During energy metabolism, glucose 6-phosphate becomes fructose half dozen-phosphate. An boosted ATP is used to phosphorylate fructose half dozen-phosphate into fructose 1,half dozen-bisphosphate past the help of phosphofructokinase. Fructose ane,6-biphosphate then splits into ii phosphorylated molecules with three carbon chains which later degrades into pyruvate.
Oxidative decarboxylation of pyruvate
Pyruvate is oxidized to acetyl-CoA and CO2 by the pyruvate dehydrogenase circuitous (PDC). The PDC contains multiple copies of iii enzymes and is located in the mitochondria of eukaryotic cells and in the cytosol of prokaryotes. In the conversion of pyruvate to acetyl-CoA, one molecule of NADH and one molecule of CO2 is formed.
Citric acid wheel
This is also called the Krebs wheel or the tricarboxylic acid cycle. When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. Once acetyl-CoA is formed, aerobic or anaerobic respiration can occur.[6] When oxygen is nowadays, the mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not nowadays, fermentation of the pyruvate molecule will occur. In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters the citric acid bike (Krebs wheel) inside the mitochondrial matrix, and is oxidized to CO2 while at the same fourth dimension reducing NAD to NADH. NADH tin exist used by the electron transport chain to create further ATP every bit part of oxidative phosphorylation. To fully oxidize the equivalent of 1 glucose molecule, two acetyl-CoA must exist metabolized by the Krebs cycle. Ii low-energy waste products, H2O and CO2, are created during this cycle.
The citric acid wheel is an viii-footstep process involving 18 unlike enzymes and co-enzymes.[6] During the cycle, acetyl-CoA (2 carbons) + oxaloacetate (4 carbons) yields citrate (6 carbons), which is rearranged to a more reactive form chosen isocitrate (6 carbons). Isocitrate is modified to become α-ketoglutarate (5 carbons), succinyl-CoA, succinate, fumarate, malate, and, finally, oxaloacetate.
The internet gain from ane cycle is 3 NADH and ane FADHii equally hydrogen- (proton plus electron)-conveying compounds and 1 high-energy GTP, which may subsequently be used to produce ATP. Thus, the total yield from 1 glucose molecule (two pyruvate molecules) is vi NADH, 2 FADHii, and 2 ATP.
Oxidative phosphorylation
In eukaryotes, oxidative phosphorylation occurs in the mitochondrial cristae. It comprises the electron send chain that establishes a proton slope (chemiosmotic potential) across the purlieus of the inner membrane by oxidizing the NADH produced from the Krebs wheel. ATP is synthesized past the ATP synthase enzyme when the chemiosmotic slope is used to drive the phosphorylation of ADP. The electron transfer is driven by the chemical energy of exogenous oxygen[1] and, with the addition of two protons, water is formed.
Efficiency of ATP production
The table beneath describes the reactions involved when one glucose molecule is fully oxidized into carbon dioxide. Information technology is causeless that all the reduced coenzymes are oxidized by the electron transport chain and used for oxidative phosphorylation.
| Step | coenzyme yield | ATP yield | Source of ATP |
|---|---|---|---|
| Glycolysis preparatory stage | −2 | Phosphorylation of glucose and fructose half dozen-phosphate uses two ATP from the cytoplasm. | |
| Glycolysis pay-off stage | 4 | Substrate-level phosphorylation | |
| two NADH | iii or 5 | Oxidative phosphorylation : Each NADH produces net one.five ATP (instead of usual 2.5) due to NADH transport over the mitochondrial membrane | |
| Oxidative decarboxylation of pyruvate | 2 NADH | v | Oxidative phosphorylation |
| Krebs cycle | 2 | Substrate-level phosphorylation | |
| half-dozen NADH | 15 | Oxidative phosphorylation | |
| 2 FADHii | 3 | Oxidative phosphorylation | |
| Total yield | xxx or 32 ATP | From the consummate oxidation of one glucose molecule to carbon dioxide and oxidation of all the reduced coenzymes. | |
Although in that location is a theoretical yield of 38 ATP molecules per glucose during cellular respiration, such conditions are generally non realized because of losses such as the cost of moving pyruvate (from glycolysis), phosphate, and ADP (substrates for ATP synthesis) into the mitochondria. All are actively transported using carriers that utilize the stored energy in the proton electrochemical gradient.
- Pyruvate is taken up by a specific, low K m transporter to bring it into the mitochondrial matrix for oxidation by the pyruvate dehydrogenase complex.
- The phosphate carrier (Flick) mediates the electroneutral exchange (antiport) of phosphate (H2POiv −; Pi) for OH− or symport of phosphate and protons (H+) beyond the inner membrane, and the driving force for moving phosphate ions into the mitochondria is the proton motive force.
- The ATP-ADP translocase (also called adenine nucleotide translocase, Pismire) is an antiporter and exchanges ADP and ATP beyond the inner membrane. The driving force is due to the ATP (−four) having a more negative charge than the ADP (−3), and thus information technology dissipates some of the electric component of the proton electrochemical gradient.
The outcome of these transport processes using the proton electrochemical slope is that more than than 3 H+ are needed to brand ane ATP. Obviously, this reduces the theoretical efficiency of the whole process and the likely maximum is closer to 28–xxx ATP molecules.[4] In do the efficiency may exist fifty-fifty lower because the inner membrane of the mitochondria is slightly leaky to protons.[vii] Other factors may also dissipate the proton gradient creating an apparently leaky mitochondria. An uncoupling protein known as thermogenin is expressed in some cell types and is a channel that can transport protons. When this protein is active in the inner membrane it short circuits the coupling betwixt the electron send chain and ATP synthesis. The potential energy from the proton gradient is not used to brand ATP just generates heat. This is particularly important in brown fat thermogenesis of newborn and hibernating mammals.
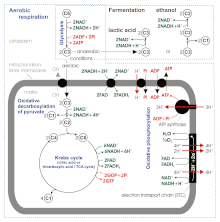
According to some newer sources, the ATP yield during aerobic respiration is not 36–38, simply simply about 30–32 ATP molecules / ane molecule of glucose [viii], because:
- ATP : NADH+H+ and ATP : FADH2 ratios during the oxidative phosphorylation appear to be not iii and 2, simply ii.5 and 1.5 respectively. Unlike in the substrate-level phosphorylation, the stoichiometry hither is difficult to constitute.
- ATP synthase produces 1 ATP / 3 H+. Even so the exchange of matrix ATP for cytosolic ADP and Pi (antiport with OH− or symport with H+) mediated by ATP–ADP translocase and phosphate carrier consumes 1 H+ / 1 ATP as a result of regeneration of the transmembrane potential changed during this transfer, so the net ratio is one ATP : 4 H+.
- The mitochondrial electron transport concatenation proton pump transfers beyond the inner membrane x H+ / 1 NADH+H+ (4 + two + four) or 6 H+ / 1 FADH2 (ii + 4).
- Then the terminal stoichiometry is
- i NADH+H+ : 10 H+ : 10/4 ATP = ane NADH+H+ : 2.5 ATP
- one FADH2 : 6 H+ : 6/4 ATP = 1 FADH2 : 1.v ATP
- ATP : NADH+H+ coming from glycolysis ratio during the oxidative phosphorylation is
- ane.5, as for FADHtwo, if hydrogen atoms (2H++2e−) are transferred from cytosolic NADH+H+ to mitochondrial FAD by the glycerol phosphate shuttle located in the inner mitochondrial membrane.
- 2.5 in case of malate-aspartate shuttle transferring hydrogen atoms from cytosolic NADH+H+ to mitochondrial NAD+
And then finally we take, per molecule of glucose
- Substrate-level phosphorylation: 2 ATP from glycolysis + 2 ATP (directly GTP) from Krebs cycle
- Oxidative phosphorylation
- 2 NADH+H+ from glycolysis: ii × ane.v ATP (if glycerol phosphate shuttle transfers hydrogen atoms) or ii × 2.5 ATP (malate-aspartate shuttle)
- 2 NADH+H+ from the oxidative decarboxylation of pyruvate and 6 from Krebs cycle: eight × ii.5 ATP
- two FADH2 from the Krebs wheel: 2 × 1.v ATP
Altogether this gives 4 + three (or 5) + xx + 3 = 30 (or 32) ATP per molecule of glucose
These figures may all the same require further tweaking every bit new structural details become available. The in a higher place value of 3 H+/ATP for the synthase assumes that the synthase translocates 9 protons, and produces three ATP, per rotation. The number of protons depends on the number of c subunits in the Fo c-ring, and information technology is at present known that this is 10 in yeast Fo[nine] and eight for vertebrates.[10] Including one H+ for the send reactions, this ways that synthesis of one ATP requires ane+x/3=four.33 protons in yeast and one+8/3 = 3.67 in vertebrates. This would imply that in human mitochondria the 10 protons from oxidizing NADH would produce 2.72 ATP (instead of two.5) and the half dozen protons from oxidizing succinate or ubiquinol would produce 1.64 ATP (instead of one.5). This is consequent with experimental results inside the margin of fault described in a recent review.[11]
The total ATP yield in ethanol or lactic acid fermentation is only 2 molecules coming from glycolysis, because pyruvate is not transferred to the mitochondrion and finally oxidized to the carbon dioxide (CO2), but reduced to ethanol or lactic acid in the cytoplasm.[viii]
Fermentation
Without oxygen, pyruvate (pyruvic acrid) is not metabolized past cellular respiration simply undergoes a process of fermentation. The pyruvate is not transported into the mitochondrion but remains in the cytoplasm, where it is converted to waste products that may be removed from the jail cell. This serves the purpose of oxidizing the electron carriers so that they tin can perform glycolysis over again and removing the excess pyruvate. Fermentation oxidizes NADH to NAD+ so it tin exist re-used in glycolysis. In the absence of oxygen, fermentation prevents the buildup of NADH in the cytoplasm and provides NAD+ for glycolysis. This waste product varies depending on the organism. In skeletal muscles, the waste material product is lactic acid. This type of fermentation is called lactic acid fermentation. In strenuous exercise, when energy demands exceed energy supply, the respiratory chain cannot process all of the hydrogen atoms joined by NADH. During anaerobic glycolysis, NAD+ regenerates when pairs of hydrogen combine with pyruvate to form lactate. Lactate formation is catalyzed by lactate dehydrogenase in a reversible reaction. Lactate can also exist used every bit an indirect forerunner for liver glycogen. During recovery, when oxygen becomes available, NAD+ attaches to hydrogen from lactate to course ATP. In yeast, the waste products are ethanol and carbon dioxide. This blazon of fermentation is known as alcoholic or ethanol fermentation. The ATP generated in this process is made by substrate-level phosphorylation, which does non require oxygen.
Fermentation is less efficient at using the energy from glucose: only 2 ATP are produced per glucose, compared to the 38 ATP per glucose nominally produced by aerobic respiration. This is because most of the energy of aerobic respiration derives from Otwo with its relatively weak, high-energy double bond.[iii] [one] Glycolytic ATP, nevertheless, is created more chop-chop. For prokaryotes to continue a rapid growth rate when they are shifted from an aerobic environment to an anaerobic surround, they must increase the charge per unit of the glycolytic reactions. For multicellular organisms, during short bursts of strenuous activity, muscle cells employ fermentation to supplement the ATP production from the slower aerobic respiration, and so fermentation may be used past a cell fifty-fifty before the oxygen levels are depleted, as is the case in sports that practise non require athletes to pace themselves, such as sprinting.
Anaerobic respiration
Cellular respiration is the process past which biological fuels are oxidised in the presence of a high-energy inorganic electron acceptor (such every bit oxygen[ane]) to produce large amounts of free energy, to bulldoze the bulk production of ATP.
Anaerobic respiration is used by some microorganisms in which neither oxygen (aerobic respiration) nor pyruvate derivatives (fermentation) is the loftier-free energy terminal electron acceptor. Rather, an inorganic acceptor such as sulfate (So4 ii-), nitrate (NO3 –), or sulfur (S) is used.[12] Such organisms are typically institute in unusual places such equally underwater caves or virtually hydrothermal vents at the bottom of the ocean.
In July 2019, a scientific study of Kidd Mine in Canada discovered sulfur-breathing organisms which live 7900 anxiety below the surface, and which breathe sulfur in order to survive. These organisms are also remarkable due to consuming minerals such as pyrite equally their food source.[13] [xiv] [15]
Run across also
- Maintenance respiration: maintenance equally a functional component of cellular respiration
- Microphysiometry
- Pasteur point
- Respirometry: research tool to explore cellular respiration
- Tetrazolium chloride: cellular respiration indicator
- Complex i: NADH:ubiquinone oxidoreductes
References
- ^ a b c d e f 1000 Schmidt-Rohr, M. (2020). "Oxygen Is the High-Energy Molecule Powering Circuitous Multicellular Life: Primal Corrections to Traditional Bioenergetics" ACS Omega 5: 2221-2233. http://dx.doi.org/10.1021/acsomega.9b03352
- ^ Bailey, Regina. "Cellular Respiration". Archived from the original on 2012-05-05.
- ^ a b c Schmidt-Rohr, Thou. (2015). "Why Combustions Are Always Exothermic, Yielding Almost 418 kJ per Mole of O2", J. Chem. Educ. 92: 2094-2099. http://dx.doi.org/10.1021/acs.jchemed.5b00333
- ^ a b c Rich, P. R. (2003). "The molecular mechanism of Keilin's respiratory chain". Biochemical Society Transactions. 31 (Pt 6): 1095–1105. doi:10.1042/BST0311095. PMID 14641005.
- ^ Reece1 Urry2 Cain3 Wasserman4 Minorsky5 Jackson6, Jane1 Lisa2 Michael3 Steven4 Peter5 Robert6 (2010). Campbell Biological science 9th Edition. Pearson Pedagogy, Inc. p. 168.
- ^ a b "Cellular Respiration" (PDF). Archived (PDF) from the original on 2017-05-x.
- ^ Porter, R.; Brand, M. (1 September 1995). "Mitochondrial proton conductance and H+/O ratio are independent of electron ship rate in isolated hepatocytes". The Biochemical Periodical (Free full text). 310 (Pt ii): 379–382. doi:10.1042/bj3100379. ISSN 0264-6021. PMC1135905. PMID 7654171.
- ^ a b c Stryer, Lubert (1995). Biochemistry (fourth ed.). New York – Basingstoke: Westward. H. Freeman and Company. ISBN978-0716720096.
- ^ Stock D, Leslie AG, Walker JE (1999). "Molecular compages of the rotary motor in ATP synthase". Scientific discipline. 286 (5445): 1700–5. doi:ten.1126/scientific discipline.286.5445.1700. PMID 10576729.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Watt, I.N., Montgomery, Yard.G., Runswick, M.J., Leslie, A.G.W., Walker, J.E. (2010). "Bioenergetic Cost of Making an Adenosine Triphosphate Molecule in Beast Mitochondria". Proc. Natl. Acad. Sci. United states of america. 107 (39): 16823–16827. doi:10.1073/pnas.1011099107. PMC2947889. PMID 20847295.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ P.Hinkle (2005). "P/O ratios of mitochondrial oxidative phosphorylation". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1706 (one–2): 1–11. doi:x.1016/j.bbabio.2004.09.004. PMID 15620362.
- ^ Lumen Dizzying Microbiology. "Anaerobic Respiration-Electron Donors and Acceptors in Anaerobic Respiration". courses.lumenlearning.org. Dizzying.com. Retrieved Nov 19, 2020.
Anaerobic respiration is the germination of ATP without oxygen. This method still incorporates the respiratory electron send chain, merely without using oxygen as the last electron acceptor. Instead, molecules such as sulfate (SO42-), nitrate (NO3–), or sulfur (S) are used as electron acceptors
- ^ Lollar, Garnet S.; Warr, Oliver; Telling, Jon; Osburn, Magdalena R.; Sherwood Lollar, Barbara (2019). "'Follow the H2o': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory". Geomicrobiology Journal. 36: 859–872. doi:x.1080/01490451.2019.1641770. S2CID 199636268.
- ^ World's Oldest Groundwater Supports Life Through Water-Rock Chemical science Archived 2019-09-x at the Wayback Machine, July 29, 2019, deepcarbon.cyberspace.
- ^ Foreign life-forms plant deep in a mine betoken to vast 'hush-hush Galapagos' Archived 2019-09-09 at the Wayback Machine, By Corey S. Powell, Sept. 7, 2019, nbcnews.com.
External links
- A detailed clarification of respiration vs. fermentation
- Kimball's online resources for cellular respiration
- Cellular Respiration and Fermentation at Clermont Higher
brennanevaithere99.blogspot.com
Source: https://en.wikipedia.org/wiki/Cellular_respiration
0 Response to "What Carbon Sources Can Yeast Cells Metabolize to Make Atp From Adp Under Anaerobic Conditions?"
Post a Comment